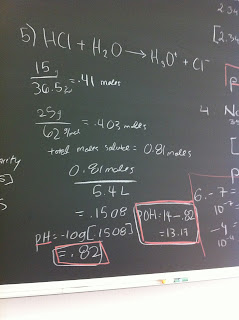Chemistry End-of-Year Bonus Assignment(s)
Create and email 3-5 minute video tutorial relating one of the topics below to the material we covered in chemistry class this year.
A tutorial is a method of transferring knowledge and may be used to give intensive instruction in some subject to an individual student or small group. Tutorials are more interactive than books and lectures, usually including pictures, diagrams, and other visual aides.
TOPICS
- Scuba Diving Tanks
- Safety Air Bags
- Breathalyzer
- Aerosol Cans
- Turbo Diesel Engines
- Ipod / Rechargeable battery
- Acid Rain
- Tarnished Jewelry
- Smoke Detector
- Carbon footprint
Other – Choose your own topic (you must ask me ahead of time)
Your tutorial must include the following information:
- An introduction stating what / how the topic is used / relates to your daily life.
- A summary of relevant background information necessary to understand the topic.
- A relationship between the topic and AT LEAST three (3) things we have discussed / studied in chemistry class.
In order for your extra-credit assignment to take affect, you must score a 10 or higher using the rubric below.
CATEGORY | 4 | 3 | 2 | 1 |
Content | Presenters show a full and thorough understanding of the topic. | Presenters show a good understanding of the topic. | Presenters show a good understanding of certain parts of the topic. | Presenters do not seem to understand the topic very well. |
Deadline | Tutorial completed and emailed before 5/30 | Tutorial completed and emailed before 5/31 | Tutorial completed and emailed before 6/1 | Tutorial completed and emailed before 6/2 |
Relevance | The relationship between class and the research topic is strong and direct in all 3 cases | The relationship between class and the research topic is strong and direct in 2 of 3 cases | The relationship between class and the research topic is strong and direct in 1 case | The relationship between class and the research topic is weak or superficial. |
Comprehension | Presenters are able to accurately answer almost all questions posed by classmates about the topic. | Presenters are able to accurately answer most questions posed by classmates about the topic. | Presenters are able to accurately answer a few questions posed by classmates about the topic. | Presenters are unable to accurately answer questions posed by classmates about the topic. |
Completion | Presenters provided a definition, a diagram, chemical reaction, and visual aides to enhance presentation | Presenters provided some of the presentation enhancements requested. | Presenters met the minimum requirements for the tutorial. | Presenters did NOT meet the minimum requirements for the tutorial. |
Chemistry End-of-Year Bonus Assignment(s)
POSTER: (Must be complete by Monday, 5/30)
Create a POSTER SIZE study guide / concept map / problem solving guide for one of the following topics:
- Naming Ionic Compounds
- Naming Covalent Compounds
- Writing & Balancing Chemical Reactions
- General Reactions Types
- Special Reaction Types
- Mole Calculations
- Mass à mols
- Particles à mols
- Volume of gas à mols
- Volume of solution à mols
- Determine Empirical & Molecular Formula
- Simple Stoichiometry & Percent Yield
- Limiting Reagent & Percent Yield
- Gas Laws
- Boyle’s Law
- Charles’s Law
- Avogadro’s Law
- Gay-Lussac Law
- Combined Gas Law
- Ideal Gas Law & Ideal Gas Constant
- Mole Fraction & Dalton’s Law of Partial Pressure
- Strong / Weak Acids & Bases
- pH calculations
- Organic Chemistry Nomenclature
MUSIC VIDEO: (Must be complete by Tuesday, 5/31)
Produce and perform in a music video presenting an original song or song parody incorporating one or more of the topics listed above.
OTHER:
Mr. Zehner is supportive of any student willing to do extra work to enhance one’s personal or one’s class’s knowledge of chemistry. If you have another idea for an extra credit assignment that will promote chemistry, help you study for the final exam, or help the class review for the final exam ask him for approval.